About VECTRON™ T500
Resistance Management
VECTRON™ T500 is an innovative insecticide developed for Indoor Residual Spraying (IRS)
to support insecticide resistance management (IRM) strategies.
01Novel Mode of Action
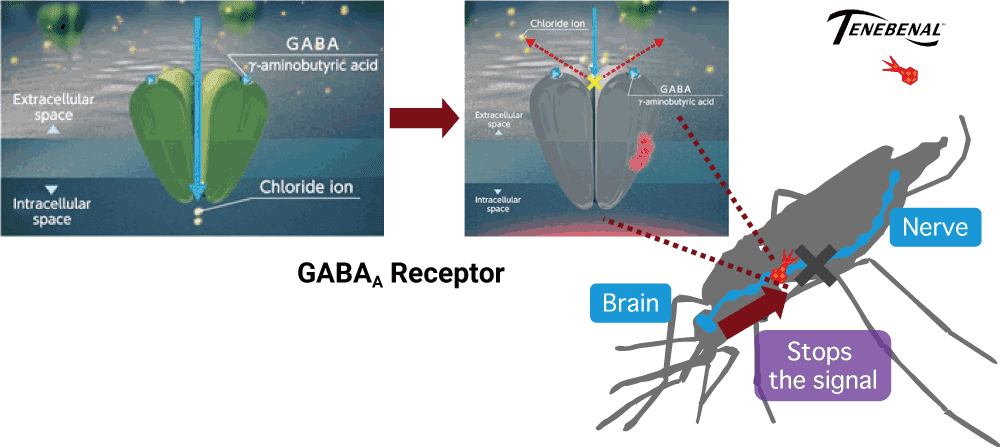
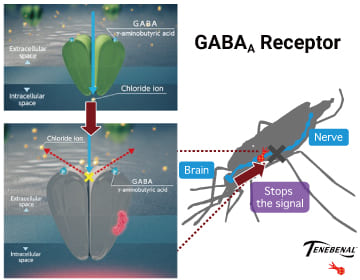
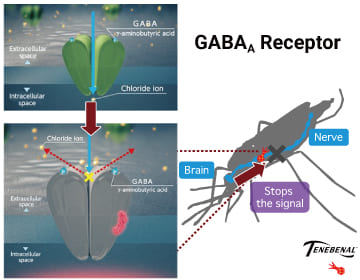
VECTRON™ T500’s active ingredient,
TENEBENAL™ affect a newly discovered target site.
Ideal for insecticide resistance management.
TENEBENAL™ is a meta-diamide insecticide which acts as an antagonist on the GABA receptors of insects. Exposure to TENEBENAL™ blocks inhibitory neurotransmission, which leads to convulsions and death of target insects. TENEBENAL™ binds to a newly discovered site on the GABA receptor which differs from conventional GABA receptor antagonists (e.g dieldrin, fipronil), resistance to conventional GABA-gated chloride channel antagonists have no effect on the insecticidal activity of TENEBENAL™, making this insecticide useful for the resistance management. Due to its unique site of action, the Insecticide Resistance Action Committee has assigned it to a new classification group (Group 30: GABA-gated chloride channel allosteric modulators).
02Long residual activity
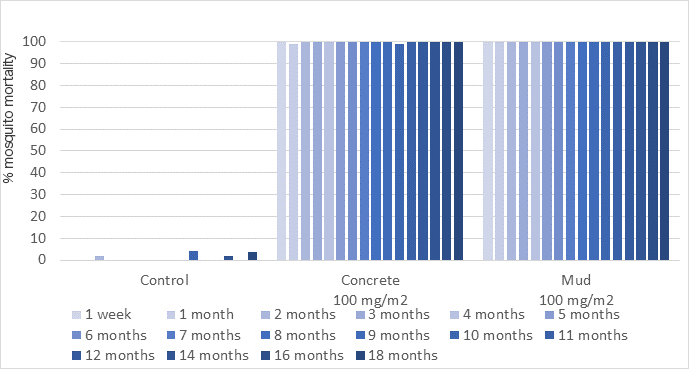
TENEBENAL™ has a low vapor pressure and is thus stable on sprayed wall surfaces for a long time, resulting in a long residual effect of VECTRON™ T500.
| Study Type | GLP Experimental Hut Trial |
|---|---|
| Location | Covè, Benin |
| Trial Year | 2019-2021 |
| Species | An. gambiae s.s. KISUMU |
| Substrate | Concrete, Mud |
| Trial Institute (+ Researcher) | CREC/LSHTM(Centre de Recherche Entomologique de Cotonou/ The London School of Hygiene & Tropical Medicine) |
| Study Condition/Method | 72-hour mosquito mortality tested in 30 min WHO cone bioassays in experimental huts |
| Study Status | Completed |
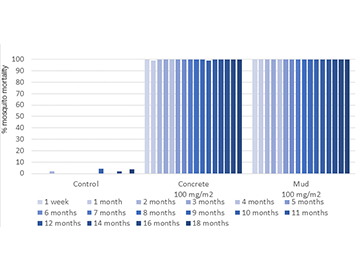
| Observation | Experimental Hut Trial has been conducted in Benin to evaluate VECTRON™ T500 residual efficacy applied on both concrete and mud hut against pyrethroid susceptible Anopheles gambiae s.s. It provided residual efficacy (>80%) through 18 months on concrete and mud substrates. |
| Explanation of Species | Susceptible |
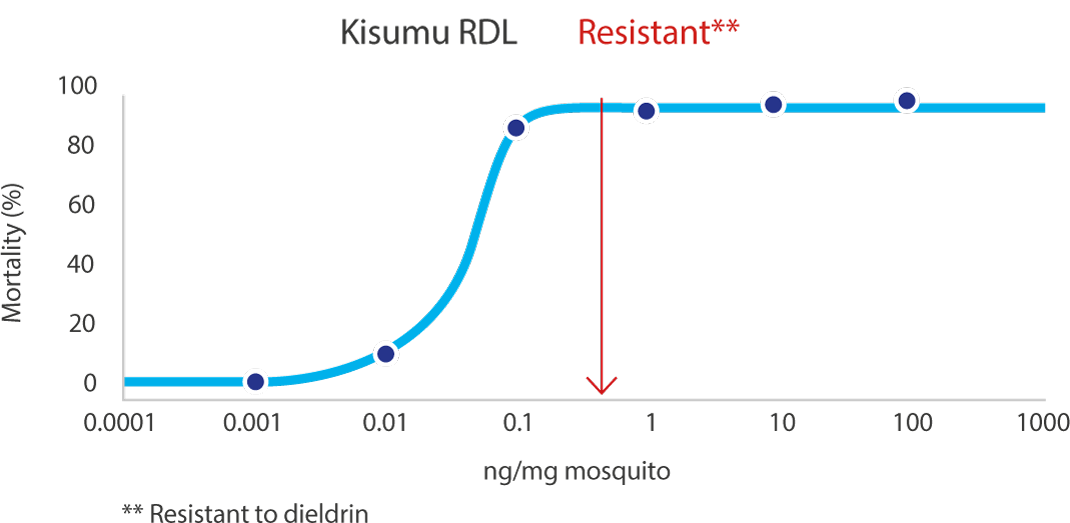
03Effective against resistant mosquito
Due to its unique mode of action, there is no record of cross resistance between TENEBENAL™ and existing insecticide classes. It therefore has a key role to play as a rotational tool as part of a proactive insecticide resistance management strategy.
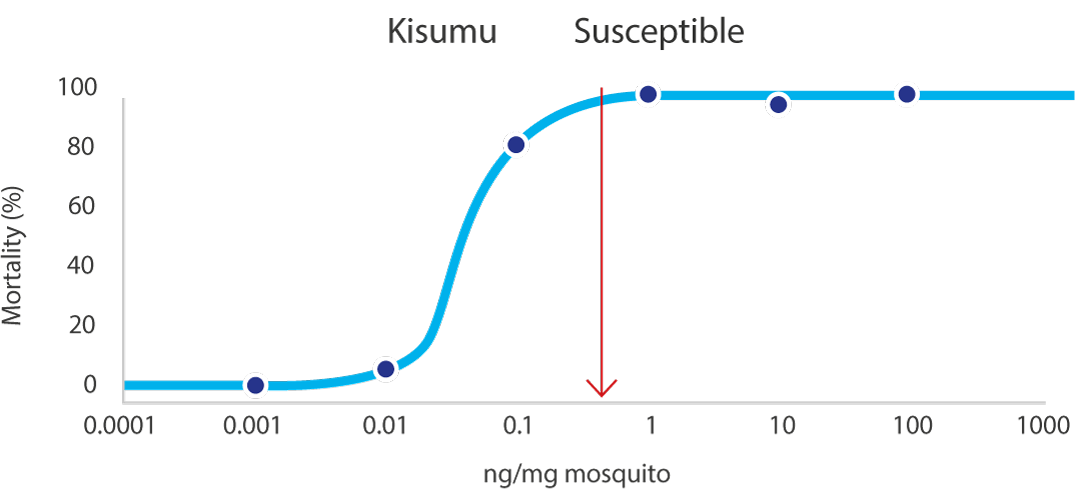
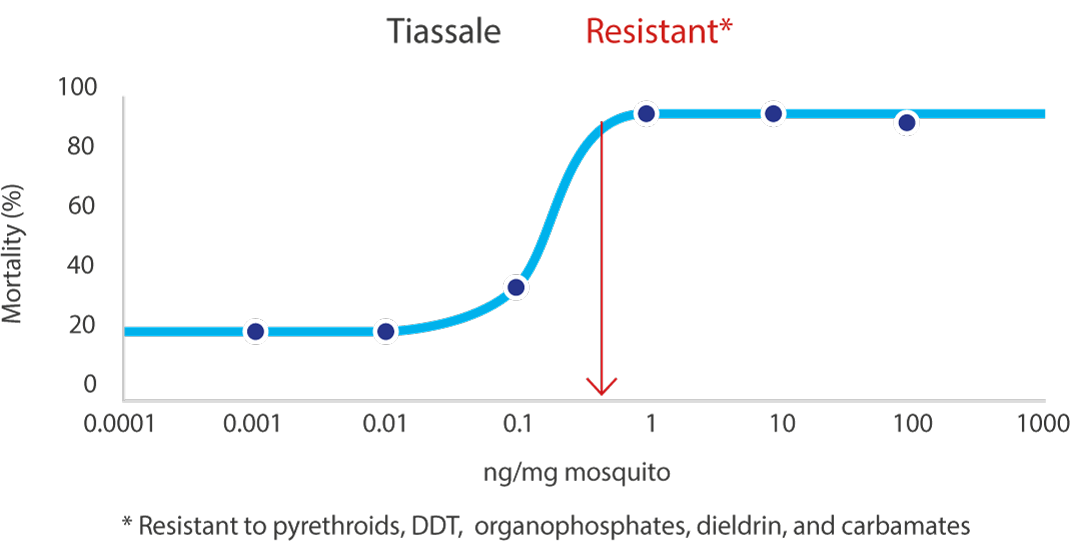
04Applicable to multiple wall types
VECTRON™ T500 has confirmed long residual efficacy when sprayed on various walls surfaces.
In laboratory trials and experimental hut studies, the application of VECTRON™ T500 demonstrated residual efficacy for six months or more against pyrethroid-susceptible and resistant mosquito strains on mud and other wall surfaces.
Various surfaces, insecticide susceptible KISUMU strain of Anopheles gambiae
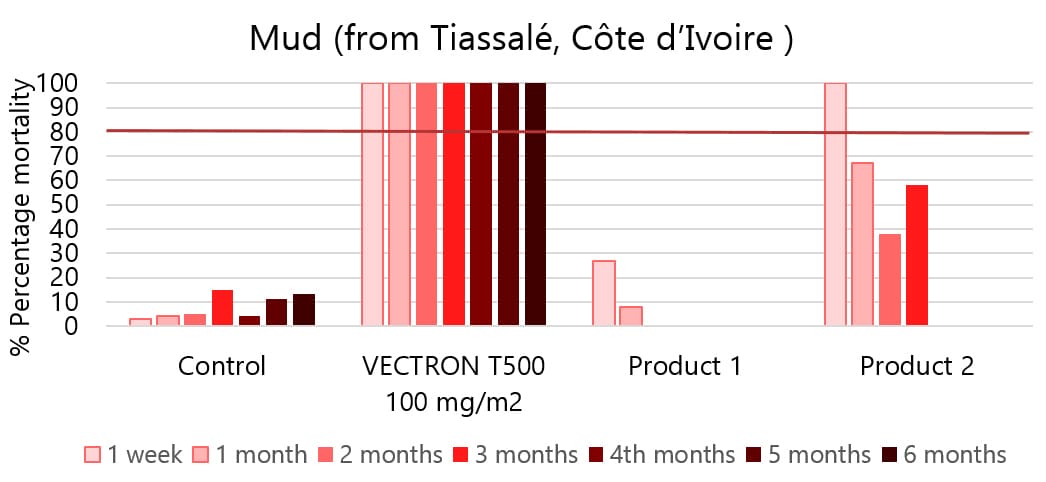
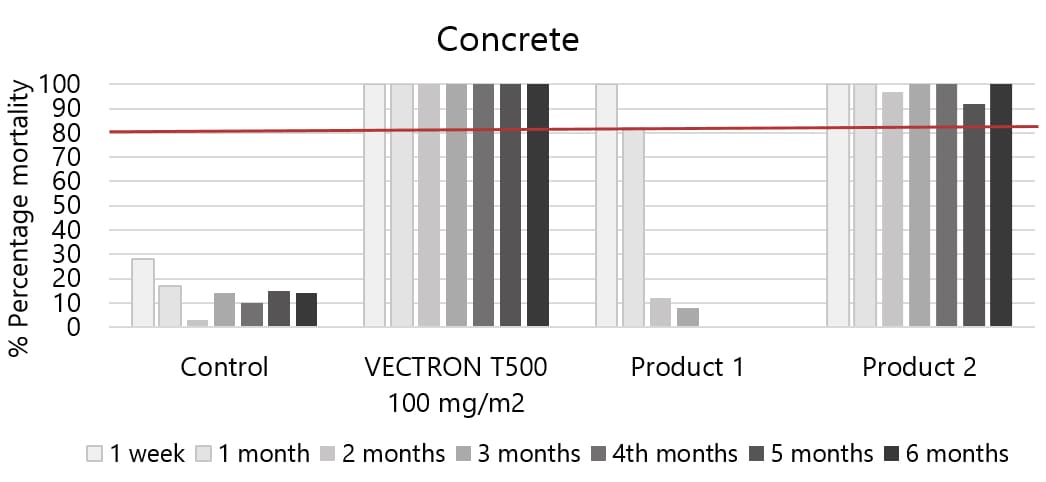
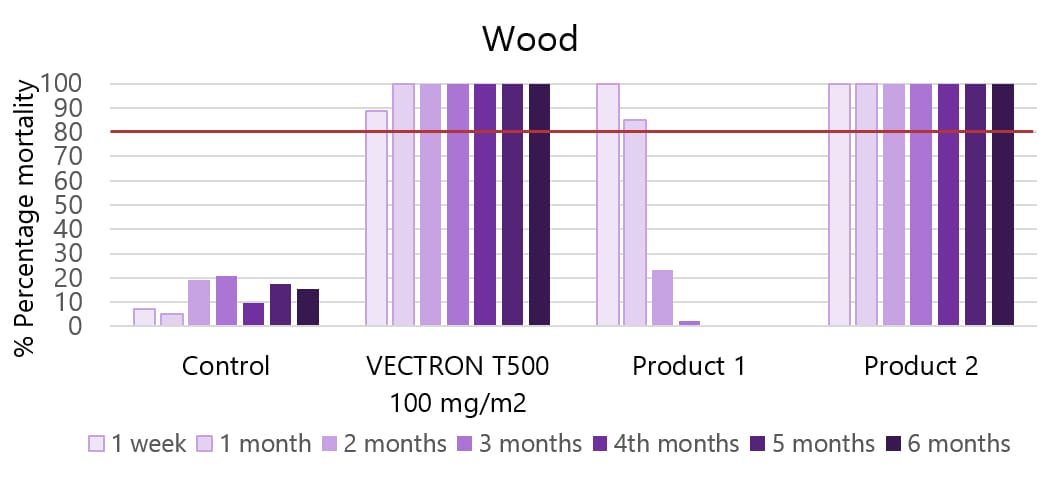
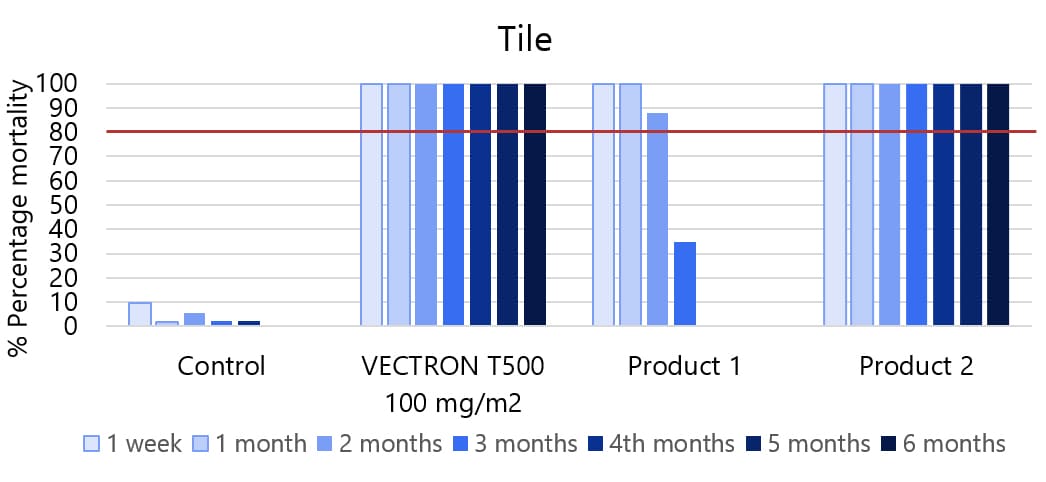
The graphs indicate Long residual activity on various surfaces in a Liverpool School of Tropical Medicine (LSTM) laboratory trial.
The red line (80%) is the WHO guidelines (2006) criterion for mosquito mortality in cone assays
05Practical and User Friendly
VECTRON™ T500 is user friendly, for residents and spray operators,
Convenient to transport due to the low weight per sachet (50g) with 100 sachets per carton. Easy to handle and add to spray tank
Easy to handle sachets and to add insecticide to a spray tank
It does not leave stains on commonly treated wall surfaces
Odourless and stainless formulation, considering community acceptance
06Low toxicity
-

Mammalian toxicity
Acute toxicity
(LD50 oral/rat)>2,000mg/kg Acute toxicity
(LD50 Dermal/rat)>2,000mg/kg Risk assessment has been determined to be acceptable for professional spray operators and residents even for children and toddlers.
Does not have any acute toxic effects oral or dermal.
-

Skin sensitization
Guinea pig Not a skin sensitizer MouseLLNA Not a skin sensitizer Does not cause skin sensitization.
Safe to be used within household and has no adverse dermal reactions.
-

Genotoxicity(TENEBENAL™)
Ames test (-/+S9) Negative Chromosomal aberration Negative Not mutagenic